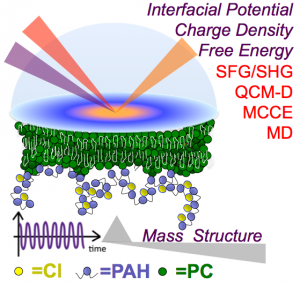
Our new paper, produced in collaboration with the Center for Sustainable Nanotechnology, pairs experiment and theory to provide, for the first time, free energies of adsorption, extent of reversibility, and charge densities of the common polycation polyallylamine hydrochloride interacting with a supported lipid bilayer. We find more than 70% charge neutralization within the attached polycation when compared to its aqueous state, and identify the binding site as consisting of contact ion groups in which three lipid phosphate moieties coordinate to the adsorbate ammonium cation. Comparison to data obtained for charged monomers reveals an unanticipated level of anticooperativity. Our results provide experimental constraints for theoretical calculations, which yield atomistic views of the structures that are formed when polycations interact with lipid membranes that will be important for predicting polycation-membrane interactions. Moreover, the mechanistic insight provided here opens possibilities for new controls over polycation-membrane interactions, can inform the design of platforms for drug delivery and transfection, and provide a means for studying how cationic patches on proteins interact with lipid membranes relevant to viral budding.